Impact of Dose Escalation of Secukinumab in Patients with Psoriatic Arthritis in Real-World Setting - ACR Meeting Abstracts

Secukinumab dosing every 2 weeks demonstrated superior efficacy compared with dosing every 4 weeks in patients with psoriasis weighing 90 kg or more: results of a randomized controlled trial* - Augustin -

Simulated concentration profiles of secukinumab 300 and 150 mg with... | Download Scientific Diagram

Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52‐week results from a Phase 3 double‐blind randomized, controlled trial - Bodemer - 2021 -

Cosentyx 150 mg solution for injection in pre-filled pen - Summary of Product Characteristics (SmPC) - (emc)

Long-term efficacy and safety of secukinumab in patients with psoriatic arthritis: 5-year (end-of-study) results from the phase 3 FUTURE 2 study - The Lancet Rheumatology
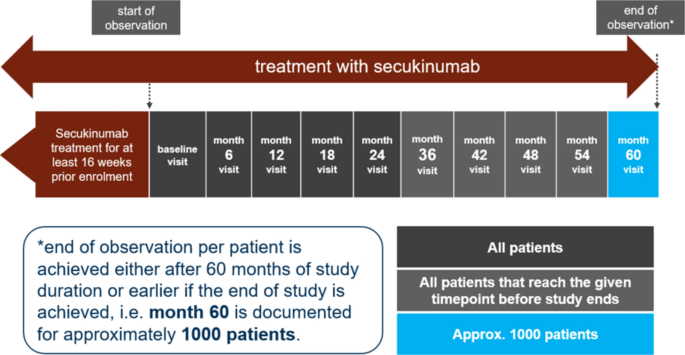
Secukinumab Use in Patients with Moderate to Severe Psoriasis, Psoriatic Arthritis and Ankylosing Spondylitis in Real-World Setting in Europe: Baseline Data from SERENA Study | SpringerLink
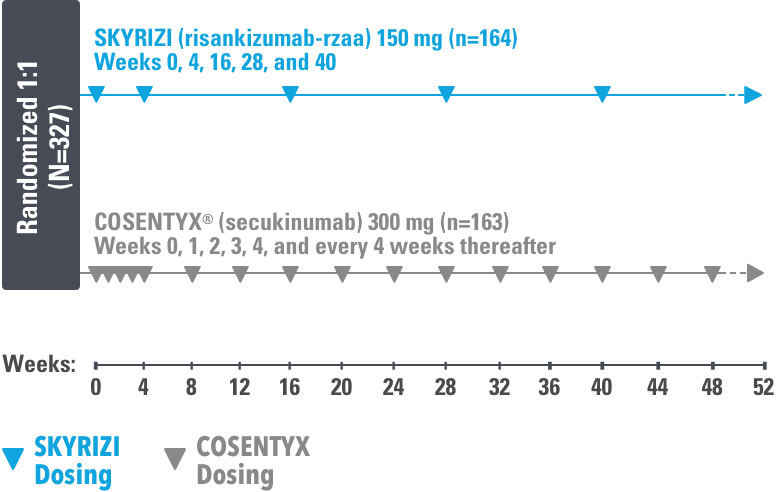
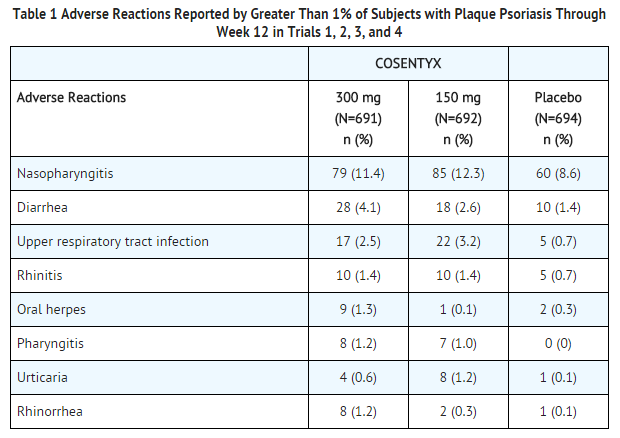
These highlights do not include all the information needed to use COSENTYX safely and effectively. See full prescribing informat

Simulated concentration profiles of secukinumab 300 and 150 mg with... | Download Scientific Diagram

These highlights do not include all the information needed to use COSENTYX safely and effectively. See full prescribing information for COSENTYX. COSENTYX® (secukinumab) injection, for subcutaneous use COSENTYX® ( secukinumab) for injection, for
![PDF] Therapeutic drug monitoring in dermatology: the way towards dose optimization of secukinumab in chronic plaque psoriasis by Rani Soenen, Rani Soenen, Zhigang Wang, Lynda Grine, Erwin Dreesen, Lisa Schots, Els Brouwers, PDF] Therapeutic drug monitoring in dermatology: the way towards dose optimization of secukinumab in chronic plaque psoriasis by Rani Soenen, Rani Soenen, Zhigang Wang, Lynda Grine, Erwin Dreesen, Lisa Schots, Els Brouwers,](https://og.oa.mg/Therapeutic%20drug%20monitoring%20in%20dermatology%3A%20the%20way%20towards%20dose%20optimization%20of%20secukinumab%20in%20chronic%20plaque%20psoriasis.png?author=%20Rani%20Soenen,%20Rani%20Soenen,%20Zhigang%20Wang,%20Lynda%20Grine,%20Erwin%20Dreesen,%20Lisa%20Schots,%20Els%20Brouwers,%20Paul%20Declerck,%20Debby%20Thomas,%20Jo%20Lambert)