Parkin, an E3 Ubiquitin Ligase, Plays an Essential Role in Mitochondrial Quality Control in Parkinson's Disease | SpringerLink

Binding to serine 65‐phosphorylated ubiquitin primes Parkin for optimal PINK1‐dependent phosphorylation and activation | EMBO reports

Model of Parkin activation by PINK1 and phospho-ubiquitin Under basal... | Download Scientific Diagram

Figure 6. | Parkin Mediates Nonclassical, Proteasomal-Independent Ubiquitination of Synphilin-1: Implications for Lewy Body Formation | Journal of Neuroscience

Structure and function of Parkin E3 ubiquitin ligase reveals aspects of RING and HECT ligases | Nature Communications

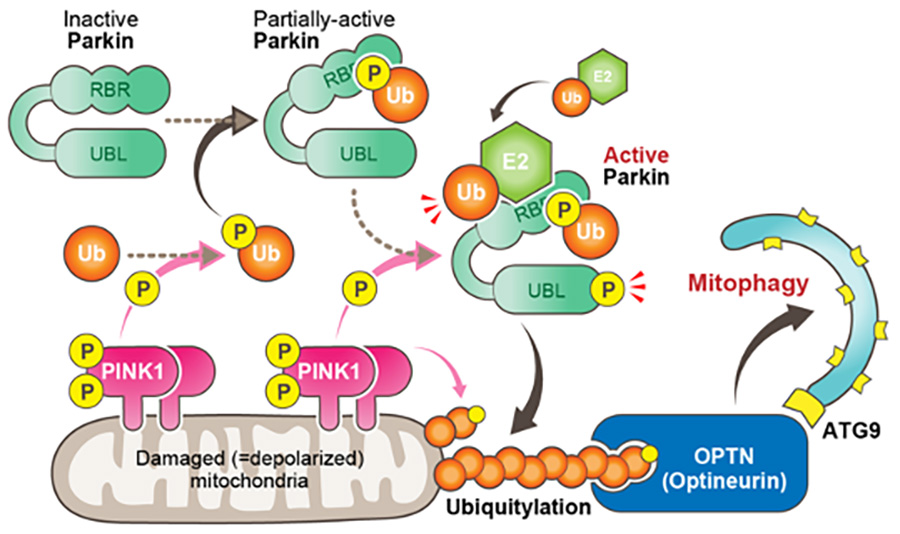
Molecular mechanisms underlying PINK1 and Parkin catalyzed ubiquitylation of substrates on damaged mitochondria. | Semantic Scholar
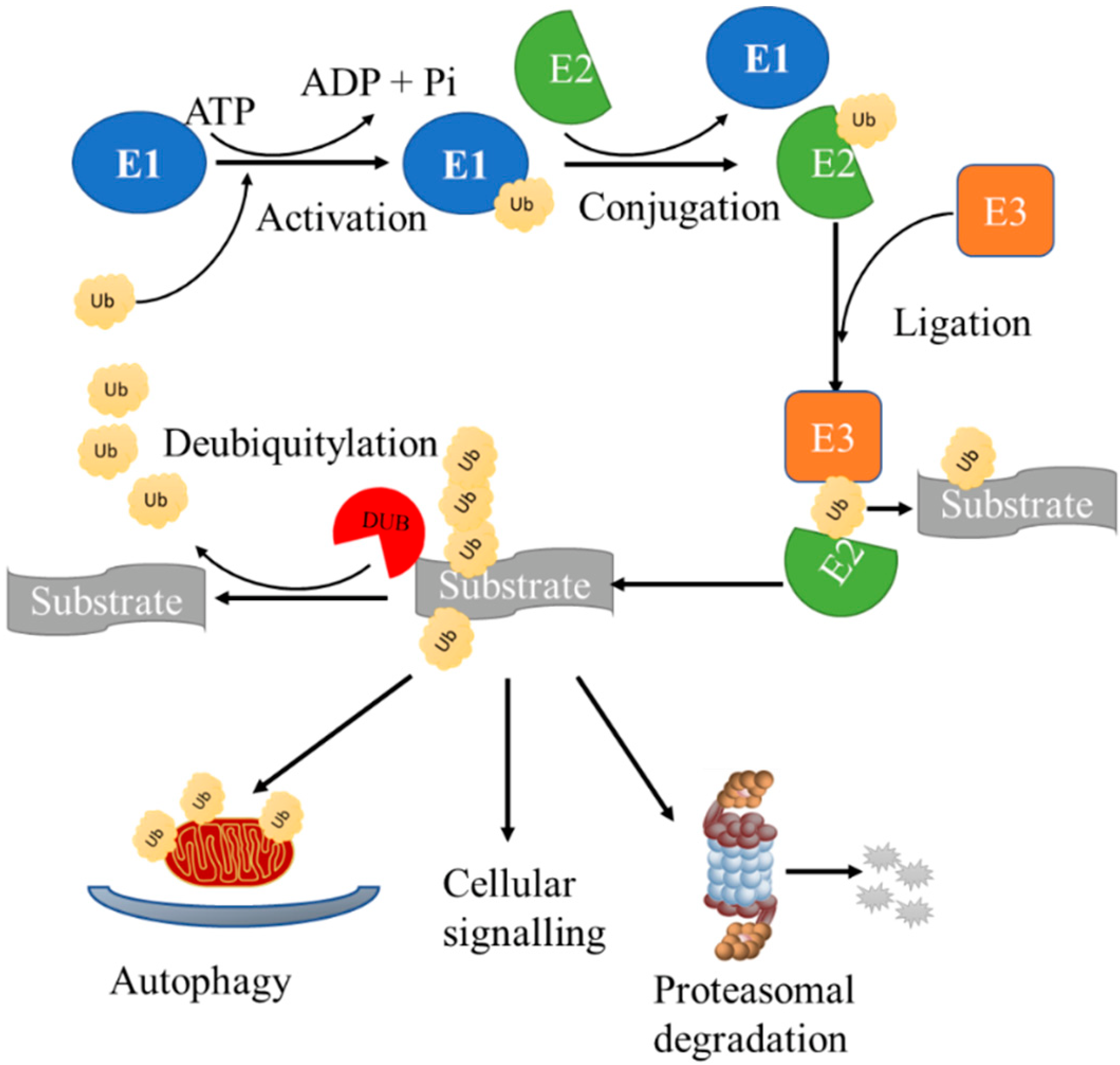
IJMS | Free Full-Text | Targeting Deubiquitinating Enzymes (DUBs) That Regulate Mitophagy via Direct or Indirect Interaction with Parkin

Parkin is an E3 ligase for the ubiquitin-like modifier FAT10, which inhibits Parkin activation and mitophagy - ScienceDirect
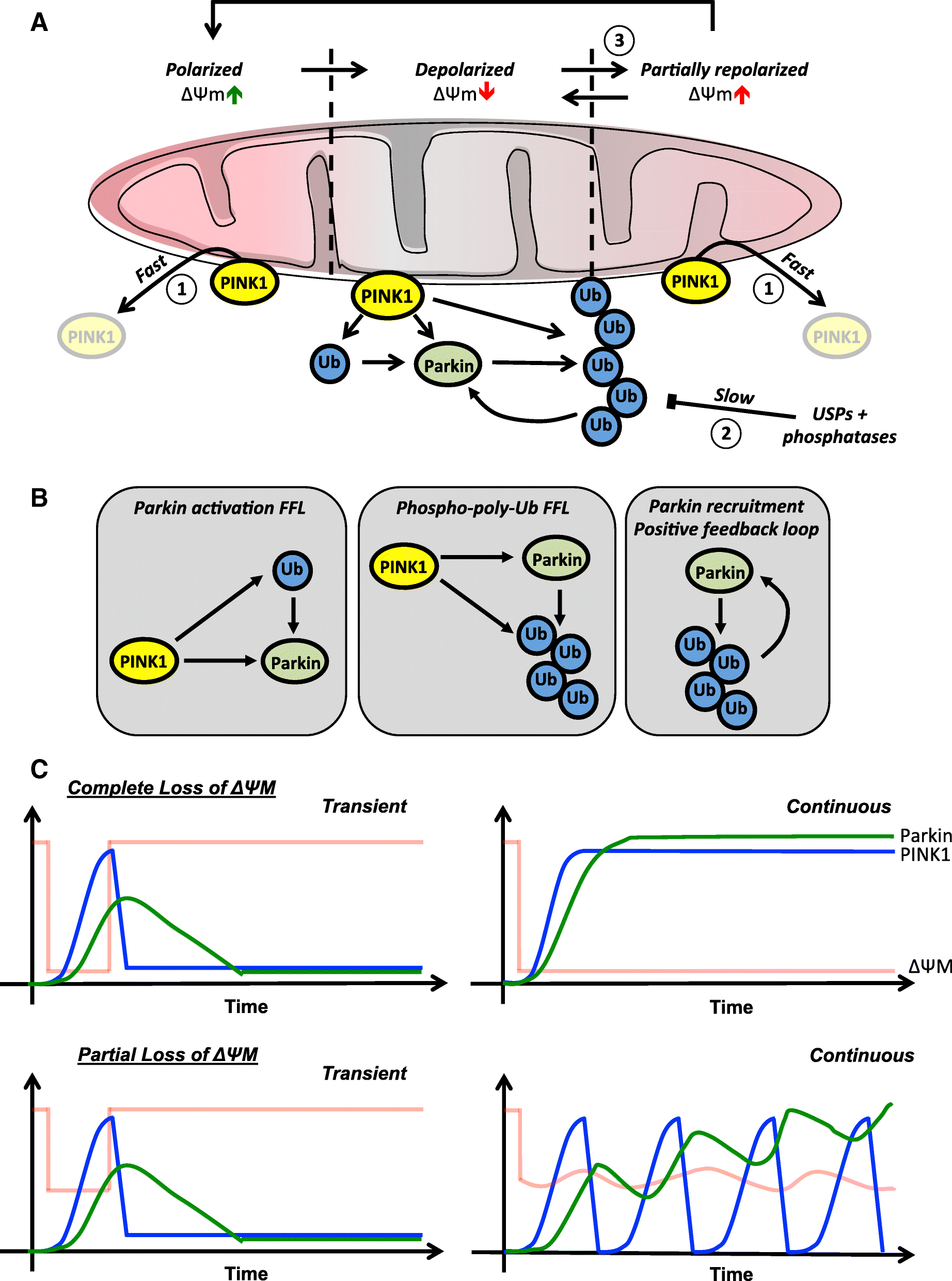
Temporal integration of mitochondrial stress signals by the PINK1:Parkin pathway | BMC Molecular and Cell Biology | Full Text
Small, N-Terminal Tags Activate Parkin E3 Ubiquitin Ligase Activity by Disrupting Its Autoinhibited Conformation | PLOS ONE

Mechanisms of PINK1, ubiquitin and Parkin interactions in mitochondrial quality control and beyond | SpringerLink

ScienceofParkinsons on Twitter: "A useful review of Parkin - an E3 Ubiquitin Ligase that plays an essential role in mitochondrial quality control in #Parkinsons https://t.co/CzK4Bcn1GO https://t.co/f3pKjDtxBX" / Twitter