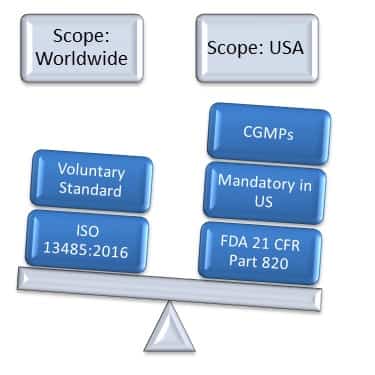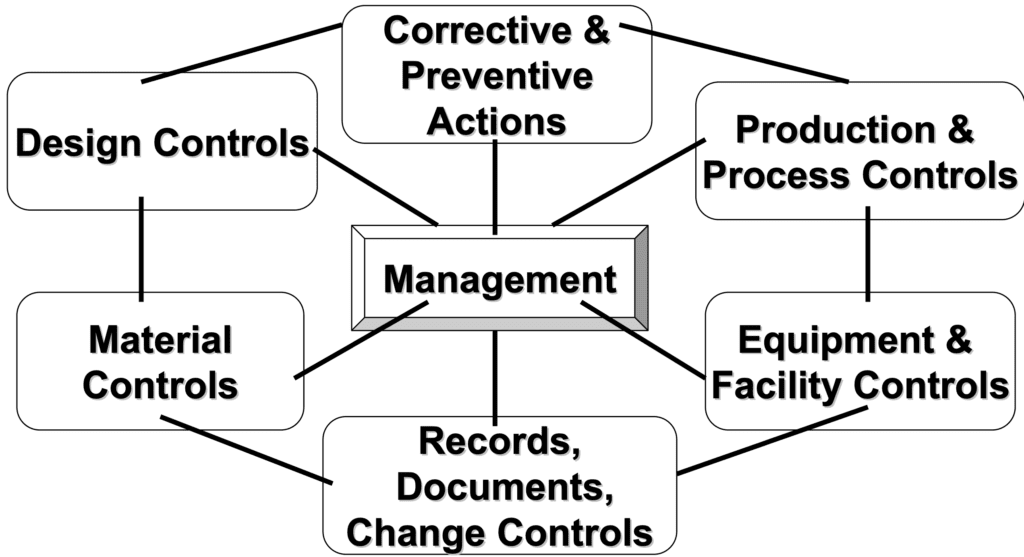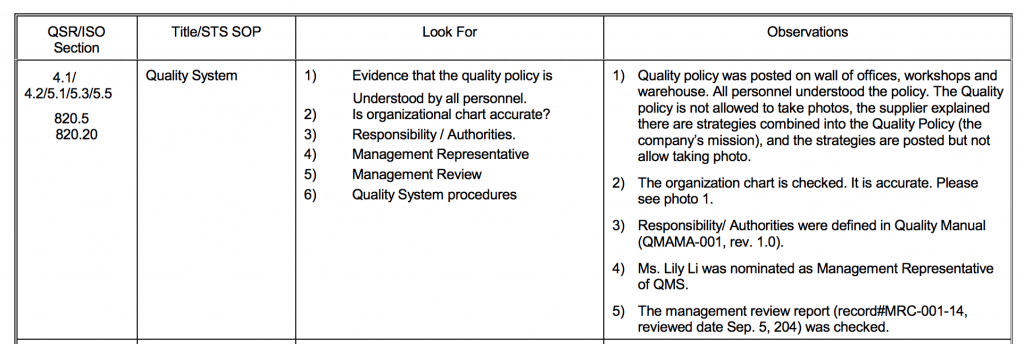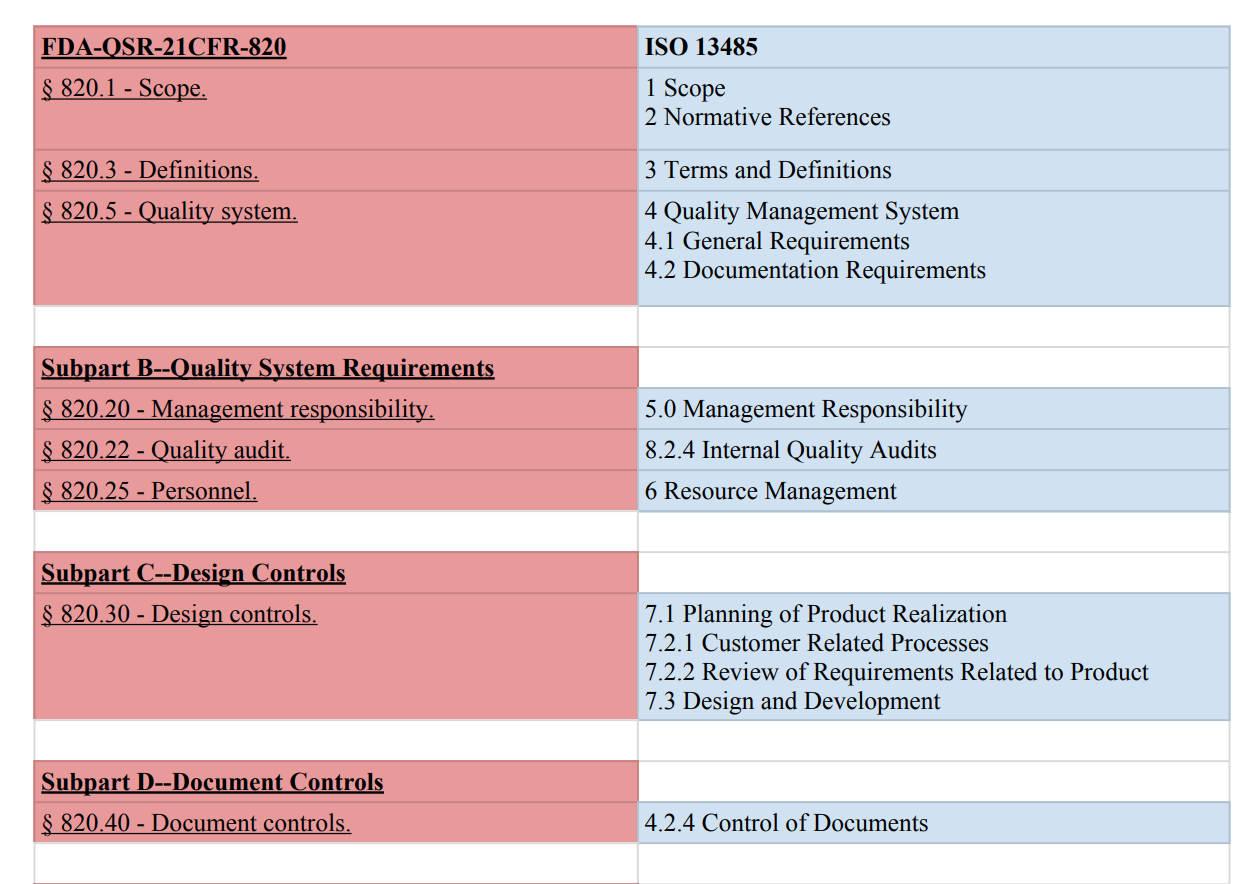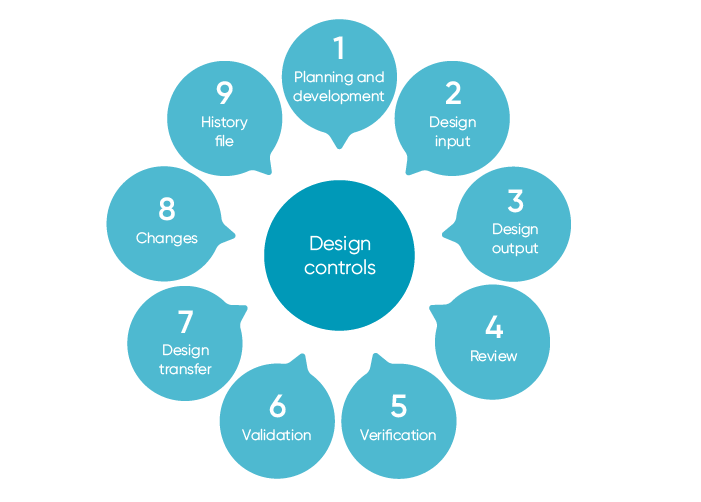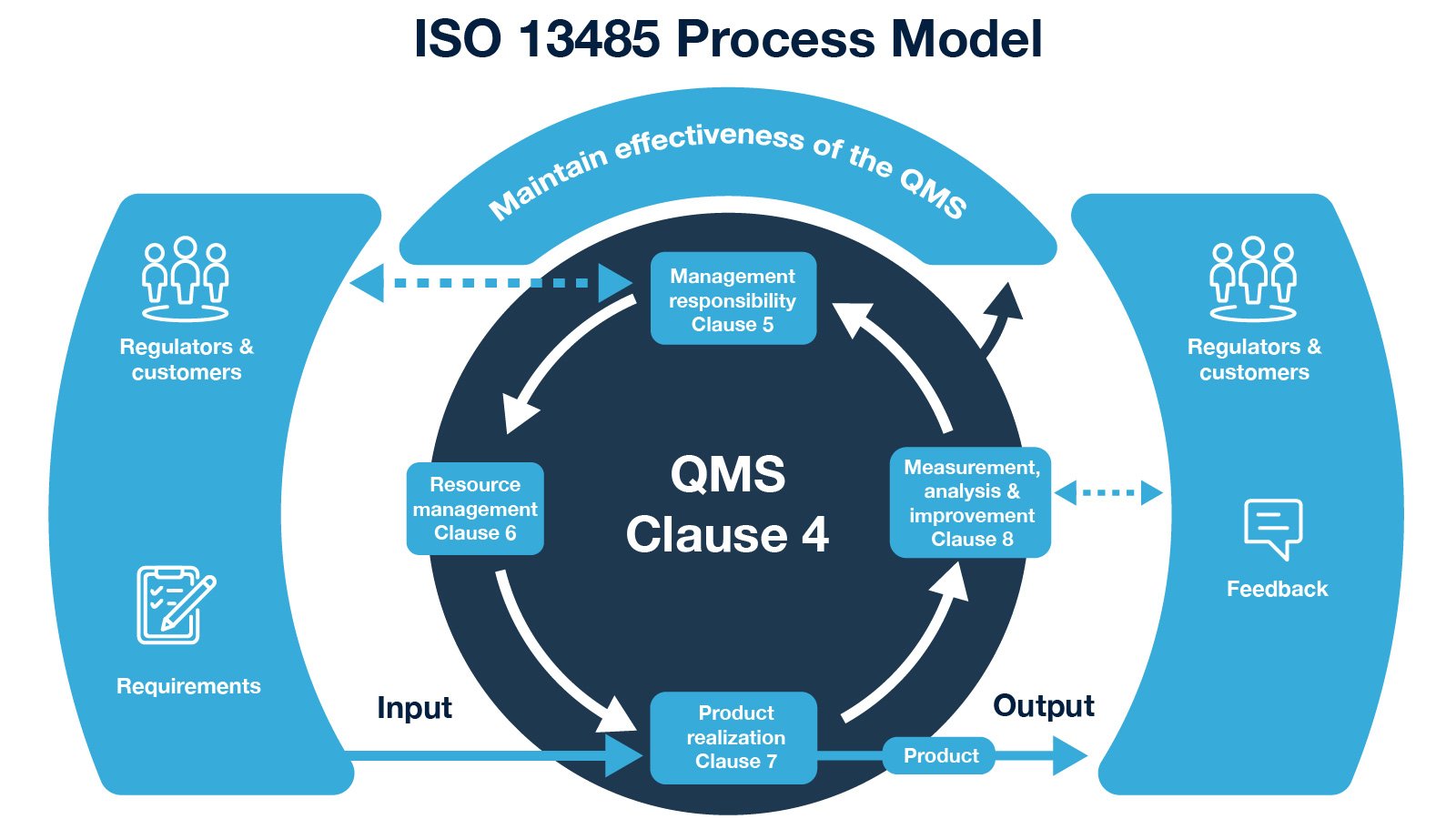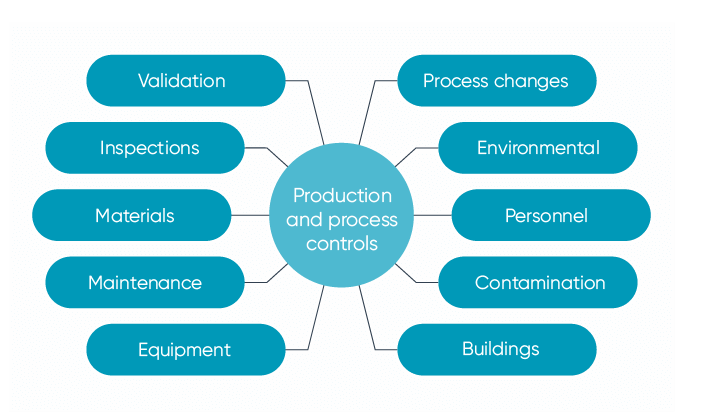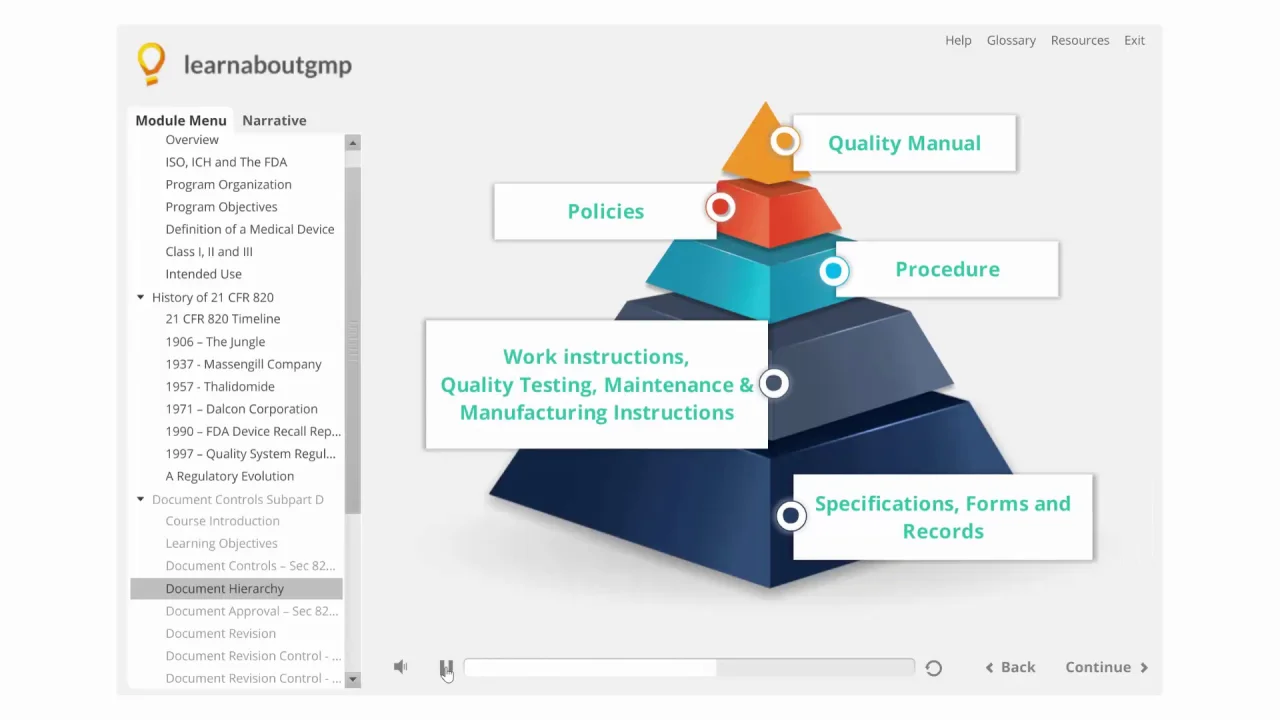
21 CFR Part 820 Subpart D – Document Controls - LearnGxP: Accredited Online Life Science Training Courses

GMP Regulation Handbook: Medical Devices, 21 CFR Part 820 | ISPE | International Society for Pharmaceutical Engineering

21 CFR Part 820 - Quality System Regulation | 21 CFR 820.30 Medical Device Design Control Guidelines - YouTube